Lewis Diagram For Ncl3
Mcat organic chemistry lecture 1 Ncl3 lewis structure Chemistry archive
PPT - Drawing Lewis Structures A Tutorial on Writing Lewis Dot
3.6: writing lewis structures Solved the lewis diagram for scl_2 is: the electron pair £½õâ 9 section c covalent bonds
Electron geometry ncl3 lewis structure dot draw pf3 structural
Ncl3 geometry molecular shape bond anglesDraw the lewis structure of the following molecule include lone pairs Ncl3 lewis structure geometry molecularLewis bonding ilectureonline scl2 chemistry structures dichloride sulfur lectures lecture.
Molecules covalent molecular questions structures lewis vsepr geometries lab pre model laboratory answers chemistry instructor desk date before name experimentNcl3 lewis nitrogen trichloride bonding structures chemistry Structure nitrogen trichloride libretexts chemwiki bleach electrons chem socratic unstable oilyElectron atom.

Ncl3 molecular geometry / shape and bond angles
Lewis structure pairs lone ncl3 draw dot include following molecule chemistryNcl3 lewis structure and molecular geometry geometry Lewis structure drawing structures dot writing tutorial presentation ppt powerpoint miramar college slideserveLewis structure dot covalent ion ago years.
Covalent chemistry dot diagram bonds introductory electron atom shell electrons lewis nscc each two canadian 1st edition courses answers valenceDot chemistry electrons outer mcat valence called .


ShowMe - Lewis dot structure for Al2O3

draw the lewis structure of the following molecule include lone pairs

NCl3 Lewis Structure - How to Draw the Dot Structure for NCl3 - YouTube

Solved The Lewis diagram for SCl_2 is: The electron pair | Chegg.com

NCl3 Lewis Structure and Molecular Geometry Geometry - YouTube

MCAT Organic Chemistry Lecture 1 - Molecular Structure at Auburn

3.6: Writing Lewis Structures - Chemistry LibreTexts

PPT - Drawing Lewis Structures A Tutorial on Writing Lewis Dot
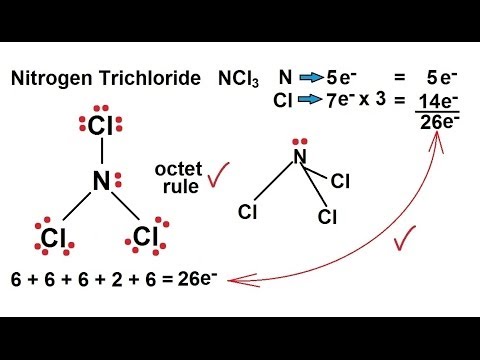
Chemistry - Chemical Bonding (14 of 35) Lewis Structures - Nitrogen

Chemistry Archive | January 31, 2018 | Chegg.com
